
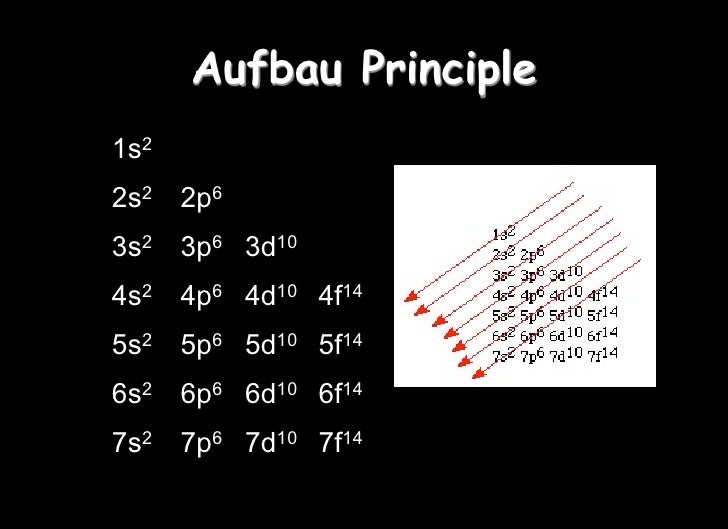
In every p subshell there are 3 p orbitals.
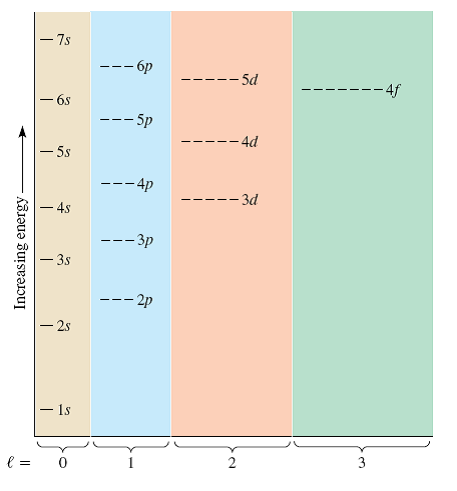
In the 2nd energy level, we have a p subshell in addition to the s subshell. This specific type of orbital is called the s orbital, and we have 1 s orbital for every s subshell. In the 1st energy level, we have 1 subshell, which basically means we have 1 type of orbital. Each orbital then has 2 electrons, which are said to have different and opposite spins. Finally, within each subshell there are individual orbitals referencing a specific region of space around the atom's nucleus. We can organize these electrons into different subshells based upon the shape of the region they occupy. The pattern that we observe results in 3 classifications as follows (there are other considerations that you will learn about later as well).Ī Shell / Energy Level is a region or set of regions that have the same energy.Īlthough we cannot predict the exact location of the electrons at any time, we can map out the regions of space that they occupy. As electrons are added to the space around the atom's nucleus they are arranged in a way as to minimize repulsions.


 0 kommentar(er)
0 kommentar(er)
